ECIS® instruments include an elevated field mode allowing for electroporation and wounding. The ECIS® wound is precisely defined, as it includes only those cells on the electrode. Additionally, with ECIS® the ECM protein coating is not scraped off and is unaffected by the current.
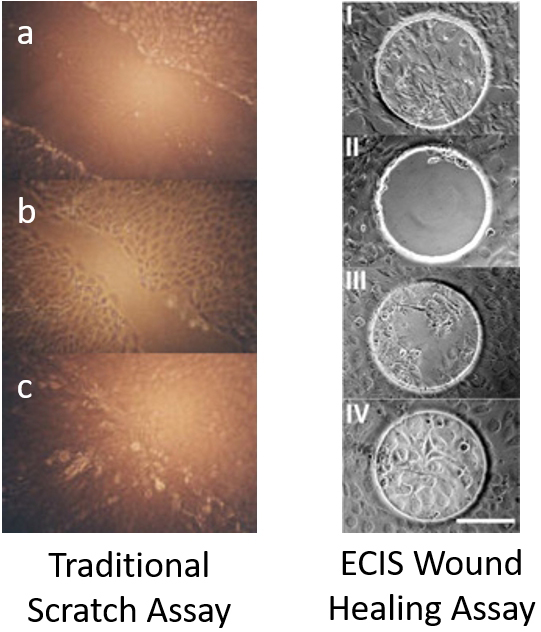
Cellular migration is an essential process that occurs in early development and maintains homeostasis from the beginning to the end of life. Due to its importance, it has become a major focal point in research. Unfortunately, the traditional methods of wound healing assays lack reproducibility and precision and have been widely criticized, until recently. Through electric cell-substrate impedance sensing (ECIS), the wound healing assay can now be measured precisely, which makes reproducing the assay very simple, and this is all done in real-time, giving the ECIS Wound Healing Assay a far greater advantage for data collection over traditional wound healing procedures.

Wound healing assays to monitor cell migration have been carried out in tissue culture for many years to estimate the migration and proliferation rates of different cells and culture conditions. The traditional “scratch” assay generally involves first growing a confluent cell monolayer, then scratching a line through the layer with objects such as toothpicks, pipette tips, rakes or even needles, which almost always destroys the absorbed proteins and extracellular matrix at the basal level. The open gap is then inspected microscopically over time as the cells move in and fill the damaged area. This "healing" can take from several hours to over a day depending on the cell type, conditions and the extent of the "wounded" region.
| Traditional Scratch Assay | ECIS Wound Healing Assay | |
|---|---|---|
| Measure Cell Migration | ||
| Precise Wounding | ||
| Real-Time Data | ||
| Automated | ||
| Highly Reproducable | ||
| Matrix Preservation |
The ECIS Wound Healing Assay replaces the traditional "scratch" assay. Instead of disrupting the cell layer mechanically with a random tip and following the migration of cells to "heal" the wound with a microscope, we employ electric signals to both wound and monitor the healing process. ECIS electrical wounding is only directed at the small population of cells in contact with the active 250 micrometer diameter ECIS electrode, producing a precise 250 micrometer wound that can be verified both with the ECIS measurement and vital staining. Unlike the traditional scratch method, the ECIS Wound Healing Assay will not affect the extracellular matrix and protein coating.
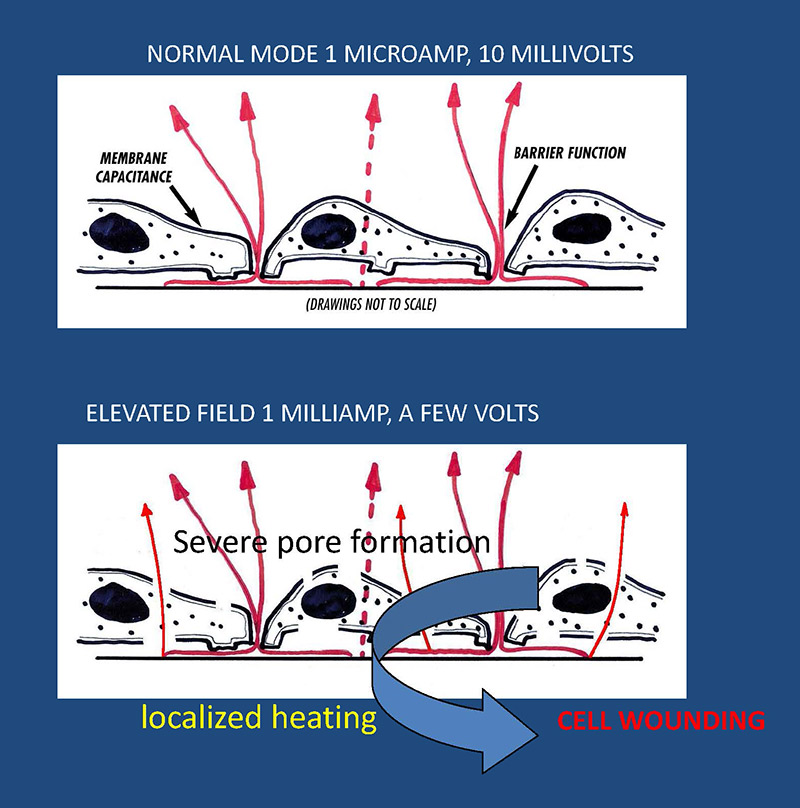
ECIS has now been modified such that automated assays of this sort can be performed. In normal ECIS measurements, a current of less than a microampere is typically used. This is undetected by the cells, and, in its measuring mode, ECIS essentially eavesdrops on cell behavior electrically. When the current is boosted by a 1000 fold to a milliampere, the resulting voltages across the cell membranes result in electroporation. If this is applied for only a few milliseconds, the cells recover and it is possible to insert impermeable molecules including DNA constructs into the cytoplasm. When the high current is applied for several seconds, cell death ensues due to severe electroporation and possible local heating effects. The ECIS wound is very well defined, as it includes only those cells on the 250 micrometer electrode. Death can be verified both with the ECIS measurement and vital staining.
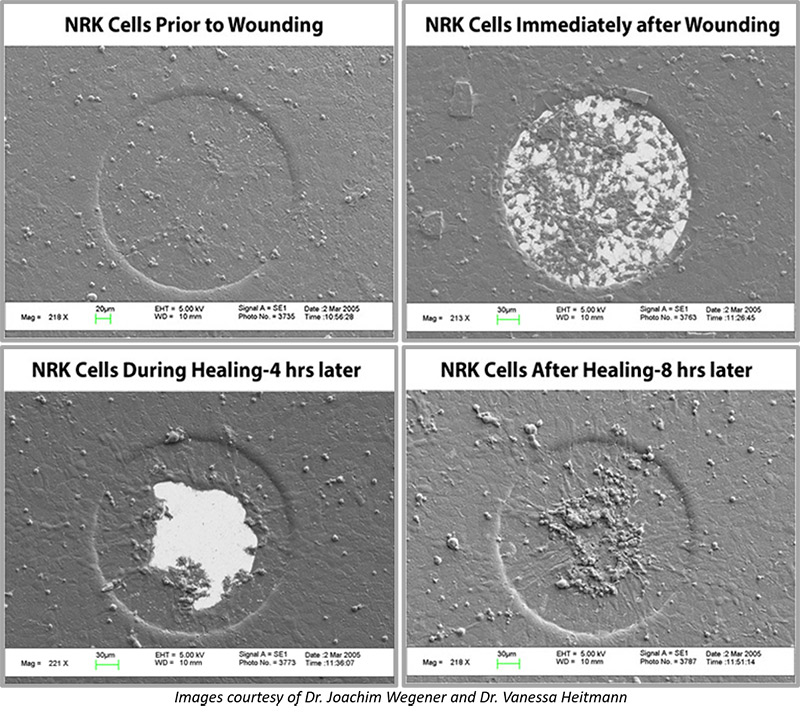
Micrographs were taken after a traditional scratch wound healing assay and an ECIS Wound Healing Assay.
The micrograph of the traditional scratch assay shows healing times of 6 hours (a), 12 hours (b), and 23 hours (c) of NRK cells following the initial wounding. A confluent layer of the NRK cells were scraped/wounded in a non-uniform pattern of unknown area using the tip of a pipette. As seen in the figure, the cells begin to migrate toward the center of the wound. Cells must be counted and compared to establish migration rate.
The micrograph of the ECIS Wound Healing Assay shows images of HUVECs as a confluent layer (I), immediately following wounding (II), 2 hours (III) and 4 hours (IV) after wounding. Note that the high electrical current completely annihilated any living cells attached to the microelectrode (II), and the rise of TEER was measured as a result of the surrounding viable cells migrating into the exposed electrodes (III, IV) (Lee, et al., 2006).
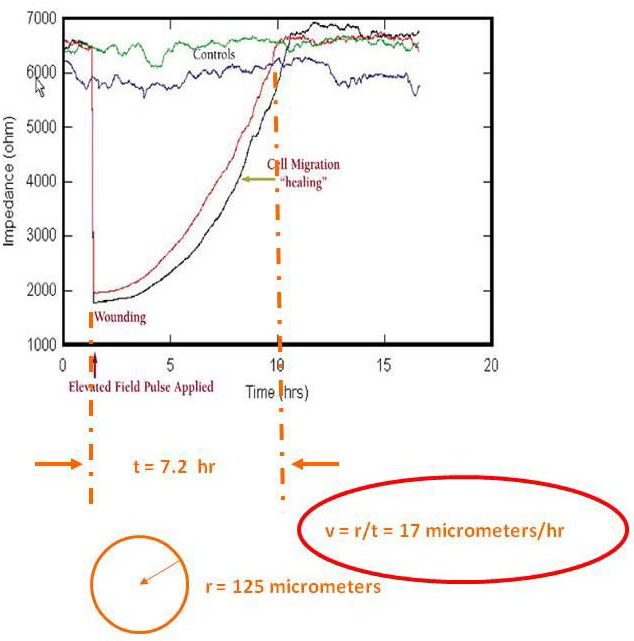
Typical ECIS data involving this assay is shown in the figure below. Here BSC1 cells were first grown as complete monolayers and the impedance traces from four confluent wells can be seen on the graph. At the arrow, an elevated field was applied to two of the wells, wounding the cells on the small electrode and causing the impedance to drop to that of an open electrode. Over time these two traces return to control values, as the healthy cells outside of the small electrode migrate inward to repopulate the wounded area and replace their dead cohorts (healing). These types of data are highly reproducible and respond to culture conditions.
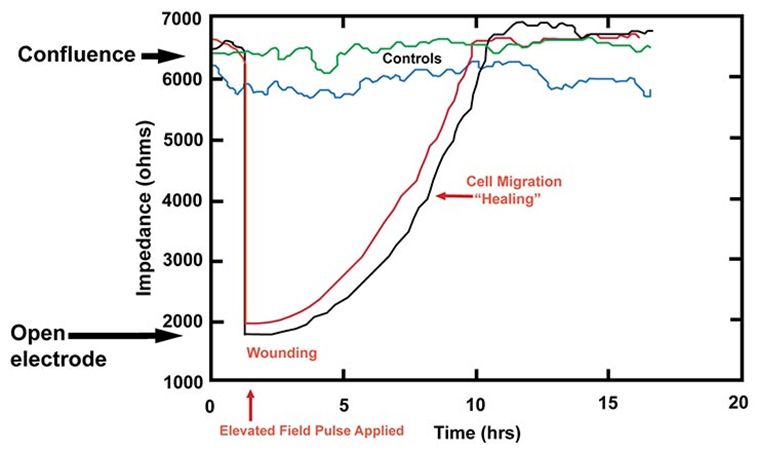
Typical ECIS data involving this assay is shown in the figure above. Here BSC1 cells were first grown as complete monolayers and the impedance traces from four confluent wells can be seen on the graph. At the arrow, an elevated field was applied to two of the wells, wounding the cells on the small electrode and causing the impedance to drop to that of an open electrode. Over time these two traces return to control values, as the healthy cells outside of the small electrode migrate inward to repopulate the wounded area and replace their dead cohorts (healing). These types of data are highly reproducible and respond to culture conditions.
The figure shows an ECIS electrode covered with MDCK cells 24 hours after the elevated field wounding took place. Note the subtle radial patterns indicated by the arrows as the cells have migrated inward. MDCK Cells wounded with ECIS Automated Cell Wounding. This is a completely automated assay requiring a minimum of labor. Both cell wounding and measurements of the subsequent healing process are carried out under computer control without opening the door of the incubator.
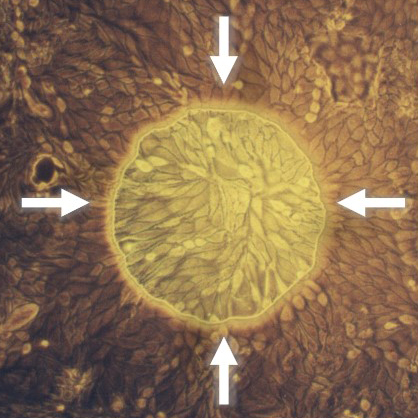
Once ECIS electrically wounds the cells, it returns to its normal mode to immediately follow the healthy neighboring cells as they migrate inward to replace the annihilated cells. Traditionally, these measurements are carried out with extensive labor including microscopy and researcher quantification. The ECIS Wound-Healing/Cell Migration Assay is a completely automated assay requiring a minimum of labor. Both cell wounding and measurements of the subsequent healing process are carried out under computer control without opening the door of the incubator.
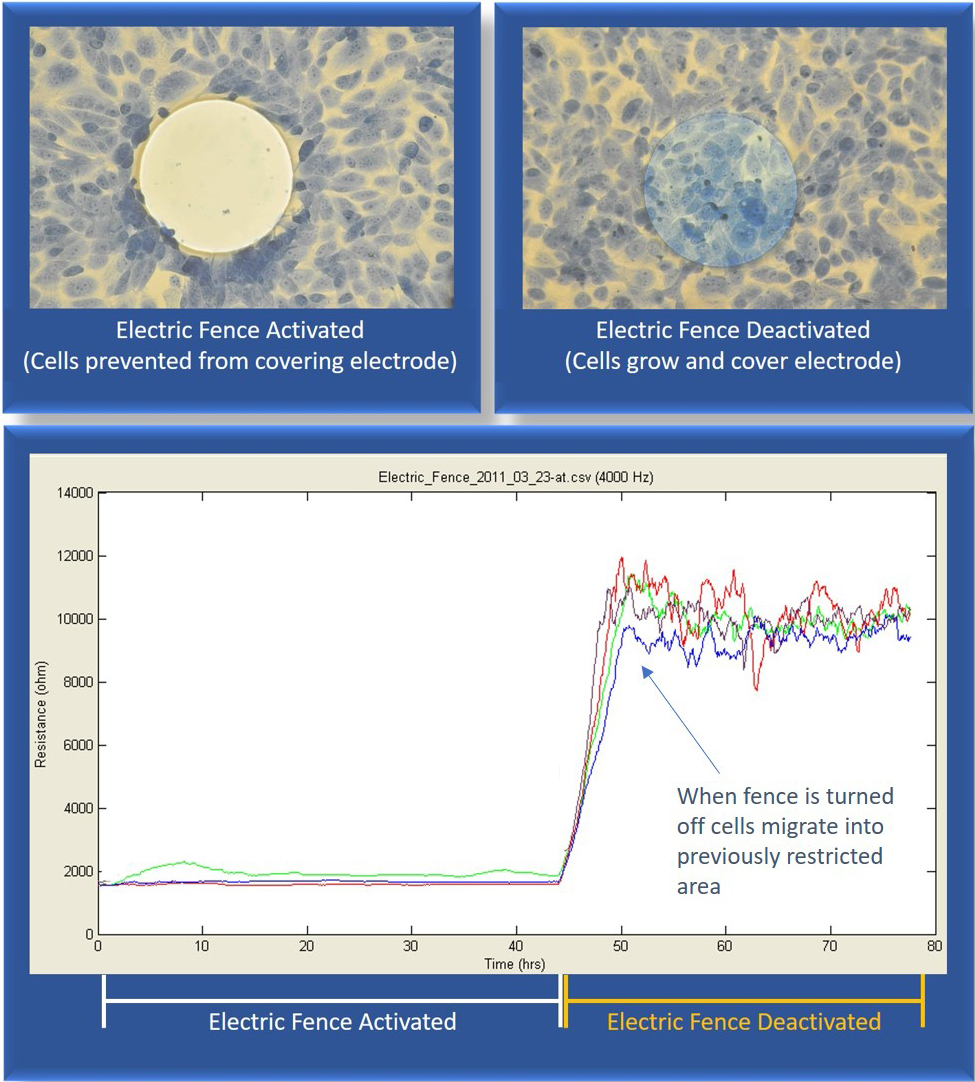
Applied Biophysics has developed a novel impedance-based technique called “The Electric Fence” to measure the rates of cell migration. The Electric Fence differs from the Wounding Assay in that it prevents the cells from actually growing on the electrode while a confluent layer develops around the electrode. When the "electric fence" is activated, a series of high field electric pulses are applied which prevent the cells from attaching and spreading onto the measurement electrode. When the electric fence is turned off, the cells in the surrounding confluent layer migrate into the open space left by the electric fence.
To view publications in wound healing/cell-migration assay please visit our publications page
© 2026 Applied BioPhysics, Inc.
185 Jordan
Road Troy, NY 12180 / Phone: 518-880-6860 / Toll Free: 866-301-ECIS (3247) / Fax: 518-880-6865