Epithelial and endothelial cells regulate the passage of molecules across their layers. Diseases, especially vascular disease, occur when this function is impaired, and so the measurement of this property in vitro is often required for research efforts. ECIS® provides a highly sensitive, real-time continuous barrier resistance measurement ideal for these types of studies.
In vivo, barriers are provided by monolayers of epithelial or endothelial cells. These cell layers play a key role regulating the free movement of molecules between different tissues and/or interstitial compartments. In many diseases, as well as in inflammation, these barriers become compromised, and hence, measuring their permeability is of considerable interest to cell biologists and the health community in general.
Most epithelial and endothelial cells types can be cultured in vitro to form monolayers where it is possible to measure the barrier function afforded by these confluent cell layers. In addition, with the right tools, dynamic changes of the layers can be followed when the cellular environment is altered by exposure to compounds or physical changes such as shear stress.
We offer different real-time approaches for electrically monitoring the barrier function (or its inverse - permeability) of cell monolayers using the ECIS® instrumentation. The approach used depends upon the degree of barrier function of the cells being studied, the throughput (number of experimental conditions) required, special experimental conditions (e.g. cells under flow) and, of course, the preference of the researcher. One other main consideration is whether one wants to monitor cells grown upon solid substrates or upon membrane filter inserts (See TEER Applications).
Monitoring cell barriers upon solid substrates with ECIS® Instrumentation
Monitoring cell barriers upon membrane filter supports with ECIS® Instrumentation
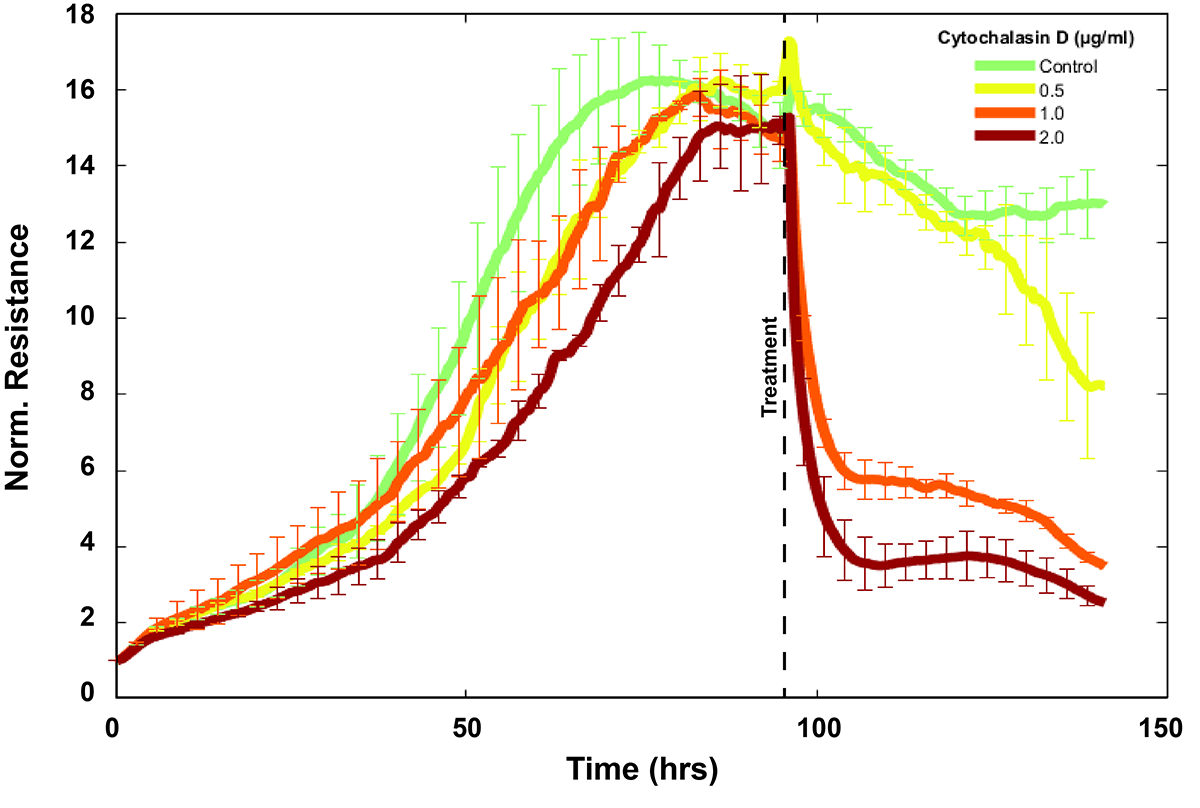
*The relative change in barrier function observed on both solid and filter shows comparable results.
Let's first consider the importance of the AC frequency used in the ECIS constant current measurement. The illustration below shows a cross section of a confluent cells layer where the path of the current is indicated by the arrows. The red arrows represent ion current flow coming from the electrode interface and moving in the solution spaces between the electrode and the basal plasma membrane and then through the paracellular passage between the cells (the barrier function). The green arrows on the other hand indicate a path of current that is possible as ECIS is an AC measurement, and current can couple capacitively through the cell membranes.
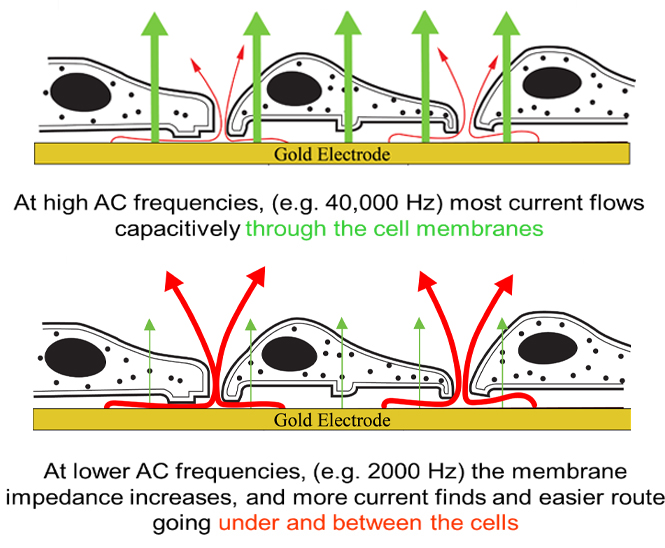
Current will always follow the path of least resistance. At high AC frequency (40,000 Hz) the impedance (capacitive reactance) of the membrane is relatively small, and current mainly capacitively couples through the insulating cell membranes with little current passing through the paracellular path. At lower AC frequency (2000 Hz in the example), on the other hand, the membrane impedance is high, and most of the current now flows under the cells and through the tight spaces between the cells (the solution path).
As a result of this difference, high frequency impedance can be used to monitor cell spreading and the establishment of a confluent layer, and low frequency impedance can be used to monitor the solution paths about the cells and hence the layer's barrier formation.
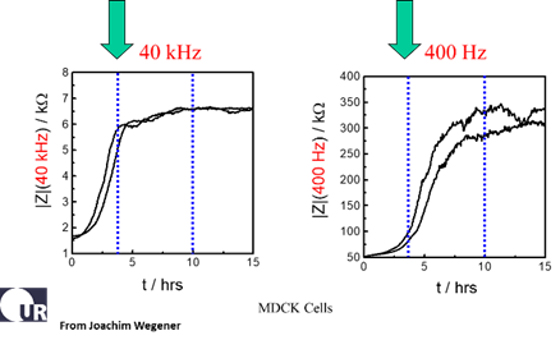
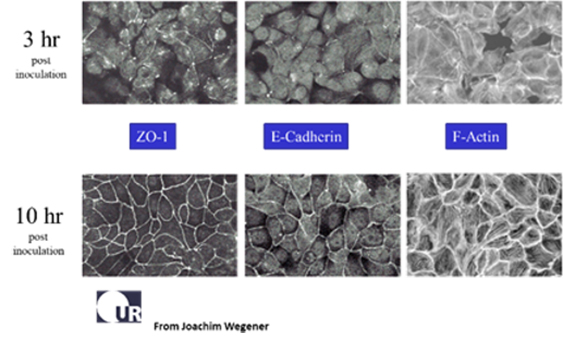
The data above report the changes of impedance in two duplicate wells following inoculation of MDCK II cells (canine kidney epithelium). The micrographs show that the cell layer is in place and confluent about 3 hours after inoculation, and this is conveyed by the plateau in the impedance at 40,000 Hz. Measuring the same wells at 400 Hz we see the formation of the barrier function is not completed until about 10 hours after inoculation. This is also confirmed microscopically using stains for the junctional proteins E-cadherin and ZO-1 (zona occludens protein) (Data courtesy of Professor Joachim Wegener, Univ. of Regensburg). For tight epithelial cells, the impedance at low AC frequencies provides a very effective measure of the layer's barrier function.
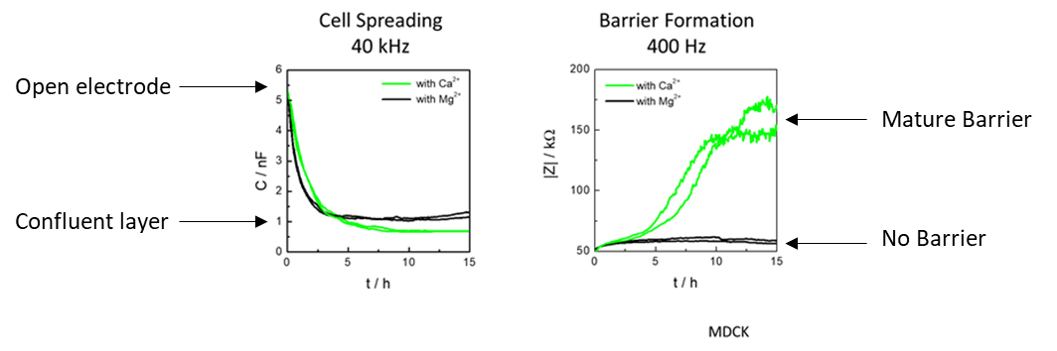
The above figure shows MDCK cells inoculated at time zero in balanced salt solutions containing only Ca2+ or Mg2+ divalent ions. Cell spreading was measured using capacitance at high frequency (40KHz) and barrier formation was measured using impedance with low frequency (400 Hz). As the graphs show, Ca+2 and Mg+2 both allow cell spreading to occur, whereas the same wells with only Ca2+ and not Mg+2 result in barrier formation.
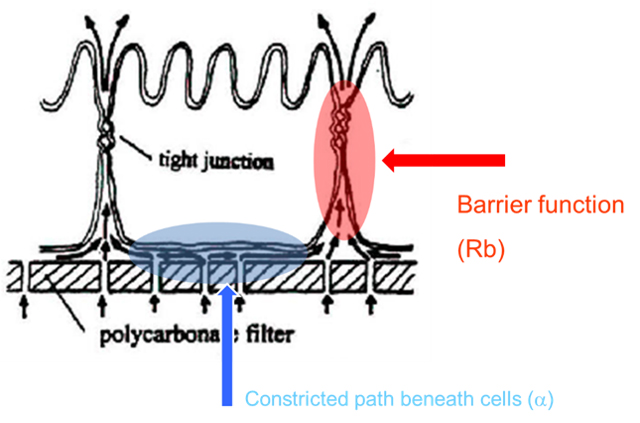
This is illustrated in the figure showing a cross section of an epithelial cell with tight junctions to its neighboring cells. In this case, we illustrate a porated filter as the cell substrate, but one could just as well have an ECIS gold electrode substrate beneath the cells. In either situation, there is a constricted space beneath the cell (shaded in blue) that is traversed before most current (or tracers) can pass through the paracellular space (the cellular barrier of interest, shaded in red).
For many epithelial cell layers, the contribution of this passage under the cell is small relative to the tight paracellular passage and does not play a major role in the measurement of barrier function. However, with cells having weaker barrier functions (e.g. some endothelial layers), this may present a complication in making a clean measurement of the paracellular path.
Fortunately, using the ECIS mathematical modeling software (Giaever and Keese), it is possible to separate these two aspects of the measurement. With this feature, time course changes in both the barrier function (Rb) and the passage beneath the cells (alpha) can be independently presented. The modeling software comes standard with the ZTheta instrumentation.
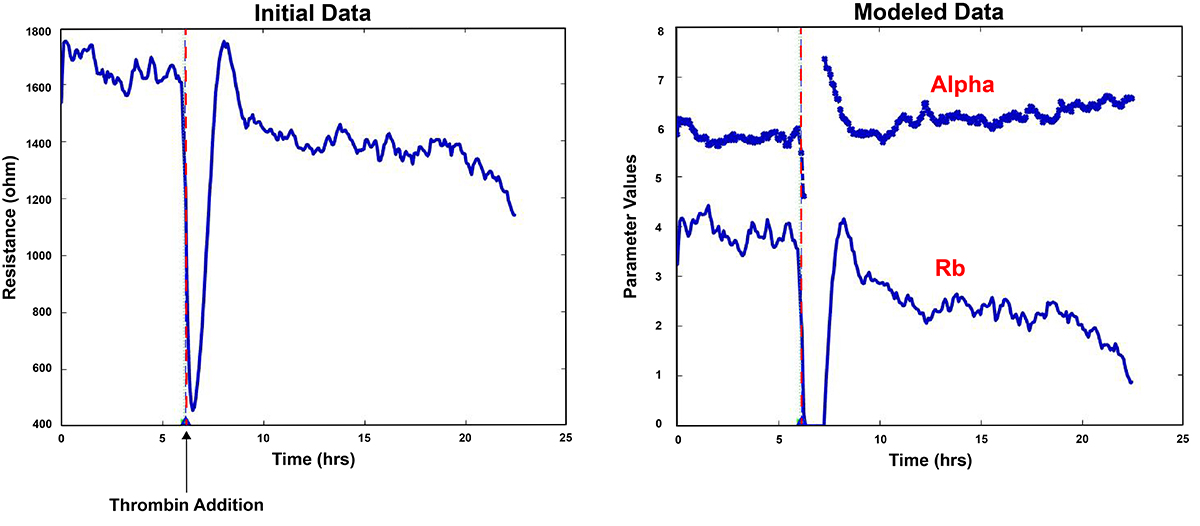
An example is shown of modeled data software, where time course changes of Rb are reported for microvascular endothelial cells exposed to thrombin. The value of alpha remains essentially constant throughout the measurements and is also plotted for comparison.
With endothelial cells, the barrier function is relatively low compared with epithelial cells with tight junctions, and the resistive portion of the ECIS impedance measured at 4000 Hz is commonly used to evaluate barrier function changes. This assay for endothelium was introduced in 1992 (Tiruppathi et.al.) and relied on the use of complex impedance measurements of endothelial cells grown upon ECIS electrodes to report just the resistive portion of the impedance. In this first study, the response of bovine pulmonary microvascular endothelial cells exposed to thrombin was evaluated (see figure below).
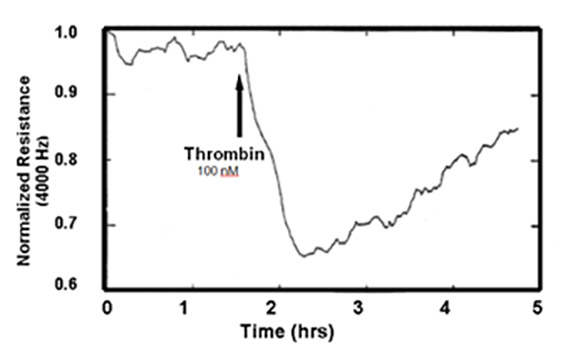
Since this first study, the resistive portion of the impedance at 4000 Hz has been used to monitor real-time changes in endothelial permeability in many laboratories, and the assay appears in many peer-reviewed publications. ECIS used in this manner has increasingly become an attractive alternative to Ussing chambers and other membrane filter measurements. In addition to its convenience in gathering barrier function data with a minimum of labor, the measurement also requires no tagged compounds and associated sampling/measuring techniques.
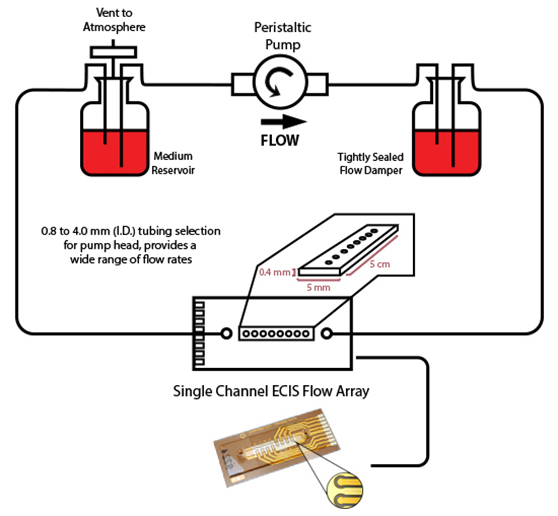
Another important feature of the ECIS system is its ability to follow the barrier function of endothelial cell monolayers as they are exposed to flow conditions. This is accomplished using ECIS flow arrays that permit measurements of cell layers subjected to shear stress as high as those experienced in vivo.
The Importance of Multifrequency Impedance Sensing of Endothelial Barrier Formation Using ECIS Technology for the Generation of a Strong and Durable Paracellular Barrier., Robilliard, Laverne,Kho, Dan,Johnson, Rebecca,Anchan, Akshata,O’Carroll, Simon,Graham, Euan (2018). Biosensors 8 (3) : 64 [Abstract]
Lo, C.M., Keese, C.R., Giaever, I., "Cell-substrate contact: Another factor may influence transepithelial electrical resistance of cell layers cultured on permeable filters", Experimental Cell Research, 250 (2): 576-580 (1999).
Tiruppathi, C., Malik, A.B., Del Vecchio, P.J., Keese, C.R., and Giaever,I., Electrical method for detection of endothelial cell shape change in real time, PNAS USA 89, 7919-7923 (1992).
Giaever, I. and Keese,C.R., PNAS USA 88, 7896 (1991).
To view publications in barrier function please visit our publications page
© 2026 Applied BioPhysics, Inc.
185 Jordan
Road Troy, NY 12180 / Phone: 518-880-6860 / Toll Free: 866-301-ECIS (3247) / Fax: 518-880-6865